-
Belite Bio Presents Results from a 24-month, Phase 2 Study of Tinlarebant in Childhood-onset Stargardt Disease at the AAO Annual Meeting
Source: Nasdaq GlobeNewswire / 06 Nov 2023 08:00:00 America/New_York
- Tinlarebant (a/k/a LBS-008) is Belite Bio’s orally administered tablet intended to slow disease progression in patients affected with Stargardt Disease (STGD1) and Geographic Atrophy (GA) in advanced Dry Age-related Macular Degeneration (Dry AMD)
- Tinlarebant was safe and well-tolerated in all subjects throughout the 24-month treatment period
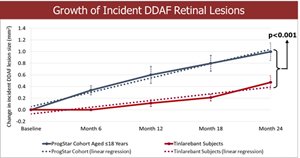
- A comparison of the 24-month DDAF lesion growth between Tinlarebant-treated subjects and ProgStar participants possessing similar baseline characteristics (aged ≤18 years) showed a sustained lower DDAF lesion growth in Tinlarebant-treated subjects over the 24-month treatment period (p<0.001)
- 42% of Tinlarebant-treated subjects (5 out of 12) did not develop atrophic retinal lesions during the 24-month treatment period
- Full enrollment of a two-year global Phase 3 study of Tinlarebant in childhood-onset STGD1 (the “DRAGON” study) is complete
SAN DIEGO, Nov. 06, 2023 (GLOBE NEWSWIRE) -- Belite Bio, Inc (NASDAQ: BLTE), a clinical-stage biopharmaceutical drug development company focused on advancing novel therapeutics targeting degenerative retinal diseases that have significant unmet medical need, presented final data from a 24-month, Phase 2 study of Tinlarebant in adolescent STGD1 (“LBS-008-CT02”) at the American Association of Ophthalmology (AAO) Annual Meeting.
“Tinlarebant’s final Phase 2 results represent a significant milestone for Belite Bio and provide additional foundational support for the work being conducted across our trials,” said Dr. Tom Lin, Chairman and CEO of Belite Bio. “The final Phase 2 data continue to demonstrate Tinlarebant’s safety profile and show a sustained lower DDAF lesion growth compared to ProgStar participants over the two-year treatment period. We hope to see similar data in the ongoing Phase 3 DRAGON study, further supporting Tinlarebant as a promising oral treatment for STGD1 patients.”
Professor John Grigg, the study’s Principal Investigator and Head Specialty of Ophthalmology at the University of Sydney and Consultant Ophthalmologist at the Sydney Children’s Hospitals Network at Westmead and Sydney Eye Hospital, presented the final study data. “We are very encouraged by the promising 24-month treatment final results from this Phase 2 study. The natural progression of childhood-onset STGD1 is characterized by a rapid visual decline and fast disease progression leading to permanent visual loss at a very young age. We are pleased that the Phase 2 final data continued to demonstrate the slowing of disease progression in the study cohort and stabilization of several structural and functional parameters, including stabilization of visual acuity.”
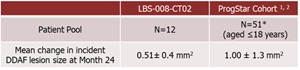
A total of 12 adolescent STGD1 subjects aged 12-18 years completed 24 months of treatment in the Phase 2 study of Tinlarebant.
Key study findings:
- Tinlarebant was safe and well-tolerated with no withdrawals due to adverse events.
- Retinal imaging showed that 5 of 12 subjects remained free of atrophic retinal lesions (referred to as definitely decreased autofluorescence or DDAF) after 24 months of Tinlarebant treatment.
- A comparison of the 24-month DDAF lesion growth between Tinlarebant-treated subjects and ProgStar participants possessing similar baseline characteristics (aged ≤18 years) showed a sustained lower DDAF lesion growth in Tinlarebant-treated subjects over the 24-month treatment period (p<0.001).
- Visual acuity was stabilized in majority of subjects during the study with a mean loss of five letters following 24 months of treatment (a loss of <10 letters is not considered clinically significant).
- A copy of the presentation slides is available here (LINK).
*Only 50 patients from ProgStar Cohort (aged ≤18) were included in the analysis due to one subject having ungradable screening FAF data.
- Strauss RW, Ho A, Muñoz B, et al. The Natural History of the Progression of Atrophy Secondary to Stargardt Disease (ProgStar) Studies: Design and Baseline Characteristics: ProgStar Report No. 1. Ophthalmology. 2016;123(4):817-28.
- Strauss RW, Muñoz B, Ho A, et al. Progression of Stargardt Disease as Determined by Fundus Autofluorescence in the Retrospective Progression of Stargardt Disease Study (ProgStar Report No. 9). JAMA Ophthalmol. 2017; 135(11):1232-1241.
About the DRAGON Study
The 2-year, Phase 3 study (DRAGON) is a Multi-Center, Randomized, Double-Masked, Placebo-Controlled Study to Evaluate the Safety and Efficacy of TinlaRebant in the Treatment of StArGardt Disease in AdOlesceNt Subjects. This study has completed recruitment (104 subjects) and one-year interim data are expected during mid to late 2024. For more information, visit clinicaltrials.gov at https://www.clinicaltrials.gov/ct2/show/NCT05244304?term=belite+bio&draw=2&rank=1).About Tinlarebant (a/k/a LBS-008)
Tinlarebant is a novel oral therapy that is intended to reduce the accumulation of vitamin A-based toxins (known as bisretinoids) that cause retinal disease in STGD1 and also contribute to disease progression in GA, or advanced Dry AMD. Bisretinoids are by-products of the visual cycle, which is dependent on the supply of vitamin A (retinol) to the eye. Tinlarebant works by reducing and maintaining levels of serum retinol binding protein 4 (RBP4), the sole carrier protein for retinol transport from the liver to the eye. By modulating the amount of retinol entering the eye, Tinlarebant reduces the formation of bisretinoids. Tinlarebant has been granted Fast Track Designation and Rare Pediatric Disease designation in the U.S., and Orphan Drug Designation in the U.S. and Europe for the treatment of STGD1.Stargardt Disease (STGD1)
STGD1 is the most common inherited retinal dystrophy (causing blurring or loss of central vision) in both adults and children. The disease is caused by mutations in a retina-specific gene (ABCA4), which results in progressive accumulation of bisretinoids leading to retinal cell death and progressive loss of central vision. The fluorescent properties of bisretinoids and the development of retinal imaging systems have helped ophthalmologists identify and monitor disease progression. Currently, there are no FDA approved treatments for STGD1.Importantly, STGD1 and GA, or advanced Dry AMD, share a similar pathophysiology, which is characterized by the excessive accumulation of bisretinoids, retinal cell death, and progressive loss of vision. Vision loss occurs slowly, despite peripheral expansion of “dead retina,” until the disease reaches the center of the eye (the macula). Therefore, Belite Bio intends to evaluate safety and efficacy of Tinlarebant in GA patients in a 2-year Phase 3 study (PHOENIX).
GA in advanced Dry Age-related Macular Degeneration (Dry AMD)
Dry AMD is a leading cause of vision loss in older adults. Geographic Atrophy, or GA, is the advanced stage of Dry AMD. Currently, there are no FDA approved orally administered treatments for GA and no FDA approved therapies for the other stages of Dry AMD other than GA. There are an estimated 20 million AMD patients in the U.S. and over 196 million patients worldwide with an estimated global direct healthcare cost of US$255 billion.About Belite Bio
Belite Bio is a clinical-stage biopharmaceutical drug development company focused on advancing novel therapeutics targeting degenerative retinal diseases that have significant unmet medical need, such as STGD1 and GA in advanced Dry AMD, in addition to specific metabolic diseases. For more information, follow us on Twitter, Instagram, LinkedIn, Facebook or visit us at www.belitebio.com.
Important Cautions Regarding Forward Looking StatementsThis press release contains forward-looking statements about future expectations and plans, as well as other statements regarding matters that are not historical facts. These statements include but are not limited to statements regarding the potential implications of clinical data for patients, and Belite Bio’s advancement of, and anticipated preclinical activities, clinical development, regulatory milestones, and commercialization of its product candidates, and any other statements containing the words “expect”, “hope” and similar expressions. Actual results may differ materially from those indicated in the forward-looking statements as a result of various important factors, including but not limited to Belite Bio’s ability to demonstrate the safety and efficacy of its drug candidates; the clinical results for its drug candidates, which may not support further development or regulatory approval; the content and timing of decisions made by the relevant regulatory authorities regarding regulatory approval of Belite Bio’s drug candidates; the potential efficacy of Tinlarebant, as well as those risks more fully discussed in the “Risk Factors” section in Belite Bio’s filings with the U.S. Securities and Exchange Commission. All forward-looking statements are based on information currently available to Belite Bio, and Belite Bio undertakes no obligation to publicly update or revise any forward-looking statements, whether as a result of new information, future events or otherwise, except as may be required by law.
Media and Investor Relations Contacts:
Jennifer Wu ir@belitebio.com
Argot Partners belitebio@argotpartners.comA chart accompanying this announcement is available at: https://www.globenewswire.com/NewsRoom/AttachmentNg/7118aed7-681e-486f-9fdc-d6af90f1c501
A table accompanying this announcement is available at: https://www.globenewswire.com/NewsRoom/AttachmentNg/ac2c1316-9c90-4f6c-94f5-d619f2123773

Comparison of the 24-month DDAF lesion growth between Tinlarebant-treated subjects and ProgStar participants
Comparison of the 24-month DDAF lesion growth between Tinlarebant-treated subjects and ProgStar participants
Comparison between Tinlarebant-treated subjects and ProgStar participants
Comparison between Tinlarebant-treated subjects and ProgStar participants